Abstract
Background: NGS panel testing can add important information for patients with cytopenia, who do not (yet) meet criteria for an MDS diagnosis. It allows clonal cytopenia of undetermined significance (CCUS) to be distinguished from an idiopathic cytopenia of unknown significance (ICUS, no marker of clonality). Currently, there is no consensus on clinical guidelines for stratification and follow-up of ICUS and CCUS. The clinical significance of repeated molecular testing to identify clonal evolution is a matter of discussion. We studied cytopenia patients with repeated NGS panel testing to elucidate clonal dynamics.
Aim: (1) Evaluate the value of repeated panel sequencing in a real life cohort of ICUS/CCUS; (2) compare the evolution pattern to MDS; (3) determine molecular features associated with progression.
Patients and Methods: 348 patients (F: 124, M: 224; median age: 70 [21-90] years) were included undergoing bone marrow examination for unexplained cytopenia. Samples were analyzed by cytomorphology, immunophenotyping, cyto- and/or molecular genetics. MDS was diagnosed according to the revised 4th edition of WHO classification 2017. CCUS and ICUS were defined according to Steensma et al., Blood 2015. Follow-up analysis (38 PB, 310 BM) was performed upon physicians’ request 3-64 months (median 16 months) after initial diagnosis. Sequencing was performed on NovaSeq (Illumina, ILMN, San Diego, CA) after hybrid capture (IDT Inc. Coralville, IA) or on the NextSeq with a TruSeq Custom Amplicon panel (ILMN). A core set of 20 myeloid genes was evaluated at both time points.
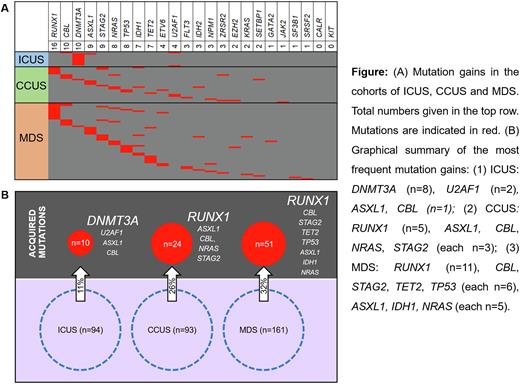
Results: At the initial examination 93 patients were classified as CCUS (mutation(s) present per definition, median age: 69 [32-90] years) and 94 as ICUS (no mutation per definition, median age 64 [21-86] years); 161 patients were classified as MDS (median age 73 [33-85] years), of whom 136 (84%) had at least one mutation. At the second testing time point, at least one mutation in an additional gene (max. 3 genes) was acquired in 24% (85/348). The frequency of mutation gains was significantly higher in CCUS (26%, 24/93) compared to ICUS (11%, 10/94, p=0.004) and similar to those already diagnosed with MDS (32%, 51/161).
Within ICUS, 7 patients acquired a sole DNMT3A mutation with a median variant allele frequency (VAF) of 3% (2-5%) not associated with disease progression. The other three patients acquired mutations in U2AF1, U2AF1+CBL or ASXL1+DNMT3A with a median VAF of 14% (6-30%) associated with disease dynamics (e.g. aplastic anemia or MDS). The most frequently acquired mutations in CCUS were found in RUNX1 (5/24), with both the acquisition of subclonal RUNX1 mutations and larger clones with 2% - 26% VAF. In addition, ASXL1, CBL, NRAS and STAG2 mutations were gained in 3 of 24 patients each. A sole acquisition of a DNMT3A mutation - as in the ICUS group - was not seen in CCUS. Median VAF of all acquired mutations in CCUS was 10% (2-79%). The pattern of acquired mutations in CCUS was similar to mutation gains in the bona fide MDS cohort (RUNX1 22%, and CBL, STAG2, TET2, TP53 12% each, Fig). Notably, mutation acquisition in CCUS was highly associated with progression; 79% (19/24) developed MDS or even AML at the time point of clonal evolution.
Conclusions: Patients with unexplained cytopenia, who do not meet diagnostic criteria of MDS (CCUS/ICUS) are a heterogeneous group in terms of molecular and clinical progression (Fig.). Cases diagnosed with ICUS had a lower risk of acquiring additional mutations than CCUS and MDS patients at later time points. CCUS patients classified as high risk based on their mutation pattern at diagnosis had a higher risk of mutation gain than non-high risk CCUS patients and resemble MDS. In patients with unexplained cytopenia the mutation pattern at initial work-up is required to differentiate between ICUS und CCUS/MDS. This determines the likelihood of progression and should guide monitoring strategies. Follow up mutation analysis can help to refine the risk of progression e.g. if blood counts change.
Disclosures
Baer:MLL Munich Leukemia Laboratory: Current Employment. Meggendorfer:MLL Munich Leukemia Laboratory: Current Employment. Kern:MLL Munich Leukemia Laboratory: Current Employment, Other: Ownership. Haferlach:MLL Munich Leukemia Laboratory: Current Employment, Other: Ownership. Haferlach:Munich Leukemia Laboratory: Current Employment, Other: Part ownership. Hoermann:MLL Munich Leukemia Laboratory: Current Employment.
Author notes
∗Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal